-
1 × $494.00
-
1 × $12,667.00
-
1 × $1,971.00
-
1 × $2,810.00
-
1 × $1,848.00
-
1 × $458.00
-
1 × $997.00
-
2 × $1,041.00
-
1 × $3,834.00
-
1 × $1,248.00
-
1 × $1,853.00
-
1 × $1,596.00
-
1 × $385.00
-
1 × $1,125.00
-
1 × $637.00
-
1 × $8,510.00
-
1 × $8,381.00
-
1 × $702.00
-
1 × $479.00
-
1 × $3,917.00
-
1 × $1,242.00
-
1 × $1,418.00
-
1 × $2,163.00
-
1 × $506.00
-
1 × $2,233.00
-
1 × $8,819.00
-
1 × $1,374.00
-
1 × $709.00
-
2 × $470.00
-
1 × $607.00
-
1 × $3,023.00
-
1 × $4,645.00
-
1 × $1,366.00
-
1 × $441.00
-
1 × $1,021.00
-
1 × $897.00
-
1 × $1,418.00
-
1 × $399.00
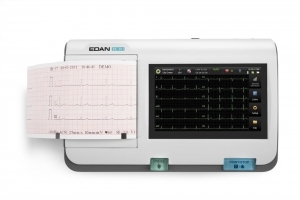
Your clinic demands top-of-the-line performance and the PageWriter Trim II cardiograph delivers. It offers the industry leading Philips 12-Lead Algorithm and produces high quality printed output for consistency of data acquisition and interpretation.
Features and Benefits
- Provides high-resolution 6.5-inch display to help you easily monitor patient ECGs
- Affordable and versatile cardiograph that makes it easy to acquire and print ECG reports in virtually any patient care setting
- Rugged, reliable model delivers years of trouble-free service, making it ideal for a demanding environment
- Designed to streamline the entire ECG testing process – to speed workflow and payback
Technical Specifications
- Patient Interface Module: Remote, microprocessor-controlled, digital module provides 5uV resolution
- Size: 6.5 inch
- Colors: 256 gray levels
- Leads off advisory: displays the label of any loose or disconnected leads/electrodes
- Heart Rate: continuous display of patient heart rate
- Print Preview: full screen preview of waveforms prior to printing
- Dimensions: 388 x 310 x 176 mm (15.3 x 12.2 x 6.9 in)
- Weight (includes battery, patient module, lead wires, alligator clips, electrode pack and paper pack): 7.38 kg (16.3 lb)
- IEC 60601-1:1988 +A1:1991 +A2:1995 General Requirement for Safety
- IEC 60601-1-2:2001 General Requirements for Safety for Electromagnetic Compatibility
- IEC 60601-2-25:1993 + A1:1999 Safety of Electrocardiographs
- IEC 60601-2-51:2003 Particular requirements for the safety and necessary performance of electrocardiographs
- UL 2601-1:L1997 US General Requirements for Safety
- CAN/CSA-C22.2 No. 601.1-M90 S1:1994 B:1996,AAMI EC11 1991:Diagnostic Electrocardiographic Devices
- CISPR11:1997 + A1:1999 + A2:2002 Radio Frequency Disturbance, Limits and Methods of Test Group 1 Class B
- JIST 1202:1998 Japanese Industrial Standard for Electrocardiographs