-
1 × $3,835.00
-
1 × $581.00
-
1 × $588.00
-
1 × $618.00
-
2 × $403.00
-
1 × $1,253.00
-
1 × $2,776.00
-
1 × $876.00
-
1 × $1,091.00
-
1 × $1,202.00
-
1 × $1,224.00
-
1 × $3,834.00
-
1 × $682.00
-
1 × $698.00
-
1 × $1,389.00
-
1 × $10,832.00
-
1 × $2,361.00
-
1 × $547.00
-
1 × $1,575.00
-
1 × $1,750.00
-
1 × $2,738.00
-
1 × $3,704.00
-
1 × $2,980.00
-
1 × $1,374.00
-
1 × $3,945.00
-
1 × $5,451.00
-
1 × $588.00
-
1 × $2,492.00
-
1 × $536.00
-
1 × $2,361.00
-
1 × $7,433.00
-
1 × $401.00
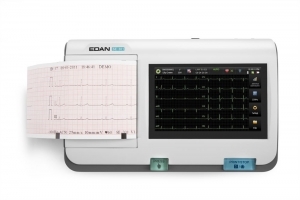
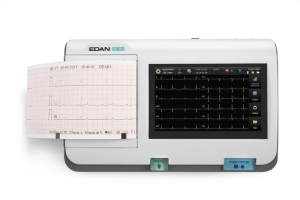
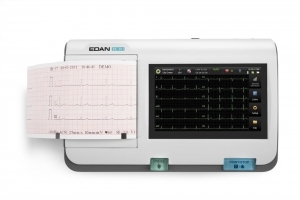
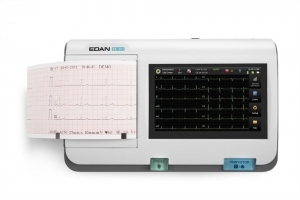
Your clinic demands top-of-the-line performance and the PageWriter Trim II cardiograph delivers. It offers the industry leading Philips 12-Lead Algorithm and produces high quality printed output for consistency of data acquisition and interpretation.
Features and Benefits
- Provides high-resolution 6.5-inch display to help you easily monitor patient ECGs
- Affordable and versatile cardiograph that makes it easy to acquire and print ECG reports in virtually any patient care setting
- Rugged, reliable model delivers years of trouble-free service, making it ideal for a demanding environment
- Designed to streamline the entire ECG testing process – to speed workflow and payback
Technical Specifications
- Patient Interface Module: Remote, microprocessor-controlled, digital module provides 5uV resolution
- Size: 6.5 inch
- Colors: 256 gray levels
- Leads off advisory: displays the label of any loose or disconnected leads/electrodes
- Heart Rate: continuous display of patient heart rate
- Print Preview: full screen preview of waveforms prior to printing
- Dimensions: 388 x 310 x 176 mm (15.3 x 12.2 x 6.9 in)
- Weight (includes battery, patient module, lead wires, alligator clips, electrode pack and paper pack): 7.38 kg (16.3 lb)
- IEC 60601-1:1988 +A1:1991 +A2:1995 General Requirement for Safety
- IEC 60601-1-2:2001 General Requirements for Safety for Electromagnetic Compatibility
- IEC 60601-2-25:1993 + A1:1999 Safety of Electrocardiographs
- IEC 60601-2-51:2003 Particular requirements for the safety and necessary performance of electrocardiographs
- UL 2601-1:L1997 US General Requirements for Safety
- CAN/CSA-C22.2 No. 601.1-M90 S1:1994 B:1996,AAMI EC11 1991:Diagnostic Electrocardiographic Devices
- CISPR11:1997 + A1:1999 + A2:2002 Radio Frequency Disturbance, Limits and Methods of Test Group 1 Class B
- JIST 1202:1998 Japanese Industrial Standard for Electrocardiographs