-
1 × $713.00
-
1 × $403.00
-
1 × $3,834.00
-
1 × $2,492.00
-
1 × $490.00
-
1 × $1,566.00
-
1 × $2,362.00
-
1 × $1,191.00
-
2 × $1,467.00
-
1 × $4,629.00
-
1 × $3,171.00
-
1 × $397.00
-
1 × $3,704.00
-
1 × $2,810.00
-
1 × $435.00
-
1 × $1,843.00
-
1 × $4,065.00
-
1 × $1,038.00
-
1 × $438.00
-
1 × $1,113.00
-
1 × $2,009.00
-
1 × $1,265.00
-
1 × $539.00
-
1 × $670.00
-
1 × $1,130.00
-
1 × $2,776.00
-
1 × $1,990.00
-
1 × $5,036.00
-
2 × $2,218.00
-
1 × $1,421.00
-
1 × $3,218.00
-
1 × $1,585.00
-
2 × $388.00
-
1 × $2,465.00
-
1 × $1,973.00
-
1 × $5,861.00
-
1 × $657.00
-
1 × $2,790.00
-
1 × $1,753.00
-
1 × $3,062.00
-
1 × $841.00
-
1 × $951.00
-
1 × $2,133.00
-
1 × $1,255.00
-
1 × $706.00
-
1 × $1,068.00
-
2 × $818.00
-
1 × $1,202.00
-
1 × $1,050.00
-
1 × $1,577.00
-
1 × $2,168.00
-
1 × $760.00
-
1 × $682.00
-
1 × $7,751.00
-
1 × $471.00
-
1 × $2,464.00
-
1 × $685.00
-
1 × $3,917.00
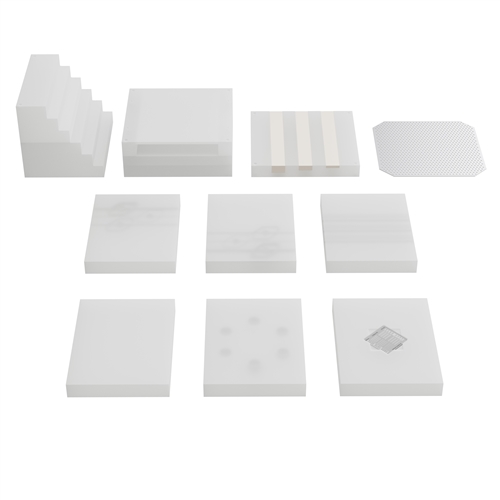
This Digital Subtraction Angiography (DSA) Phantom follows the recommendation in Report No. 15 by the AAPM (American Associationof Physicists in Medicine) – Digital Radiology / Fluorography Task Group of the Diagnostic X-Ray Imaging Committee. Phantom is designed toevaluate digital functions of DSA systems and can be used to check: contrast range, resolution, linearity, uniformity, amplifier dynamic range,registration accuracy and subtraction effectiveness. Its modular construction allows setting up the desired test configuration in a very easy andaccurate manner.
Features and Benefits
- Complies with:
- AAPM Report No. 15 – Performance Evaluation and Quality Assurance in Digital Subtraction Angiography – Diagnostic X-Ray Imaging Committee Digital Radiography / Fluorography Task Group – May 1985
- IEC 61223-3-1
- CE certified
- The manual provides detailed guidelines for carrying out each test, results assessment and registration.
- Section I and II:
- Step Wedge module, 6 step wedges (each 25.4 mm high) to test dynamic range. Can be folded into 203.2 x 203.2 x 76.5 mm solid block.
- Section III:
- Slot block with a space to fit other test modules.
- Registration Plate
- Made of aluminium with an array of 3.18 mm holes.
- Bone Section
- With three PTFE strips 25 mm in width and 5, 10 and 15 mm in thickness.
- High Contrast Resolution Section
- Containing pattern for line pair resolution evaluation (from 0.6 to 5.0 LP/mm).
- Thanks to the dedicated recesses, test pattern can be placed in different orientations.
- Blank insert
- Used with other modules forms a mask plate.
- Linearity Insert
- Contains 6 iodine areas (19 mm diameter) of different iodine thickness and concentration: 0.5, 1.0, 2.0, 4.0, 10.0 and 20.0 mg/cmІ.
- Low Contrast Artery Insert
- Containing 3 sets of analogue blood vessel group (diameters 0.5, 1.0, 2,0 and 4.0 mm), each vessels group includes different iodine concentrations: 2.5, 5.0 and 10.0 mg/cc.
- 150 mg/ml Iodine Artery Insert
- 3 sets of analogue blood arteries (thickness 1.0, 2.0, 4.0 mm) each includes arteriarctial and arterial aneurysm whose sizes are 1/4, 1/2 and 3/4 of artery diameter.
- 300 mg/ml Iodine Artery Insert
- 3 sets of analogue blood arteries (thickness 1.0, 2.0, 4.0 mm) each includes arteriarctia and arterial aneurysm whose sizes are 1/4, 1/2 and 3/4 of artery diameter.
- Carrying Case
Technical Specifications
- Size:
- Section I and II:
- 6 step wedges (each 25.4 mm High)
- Can be folded into 203.2 x 203.2 x 76.5 mm solid block.
- Section III:
- Outer Size: 203.2 x 203.2 x 76.5 mm
- Slot Size: 203.2 x 152.4 x 25.4 mm
- Registration plate:
- 203.2 x 203.2 x 1.5 mm
- Bone section:
- 203.2 x 203.2 x 25.4 mm
- High Contrast Resolution Section
- 203.2 x 203.2 x 25.4 mm
- Blank Insert
- 203.2 x 152.4 x 25.4 mm
- Linearity Insert
- 203.2 x 152.4 x 25.4 mm
- Low Contrast Artery Insert
- 203.2 x 152.4 x 25.4 mm
- 150 mg/ml Iodine Artery Insert
- 203.2 x 152.4 x 25.4 mm
- 300 mg/ml Iodine Artery Insert
- 203.2 x 152.4 x 25.4 mm
- Section I and II: