-
1 × $561.00
-
1 × $17,833.00
-
1 × $818.00
-
1 × $515.00
-
1 × $385.00
-
1 × $897.00
-
1 × $1,309.00
-
1 × $509.00
-
1 × $1,725.00
-
1 × $4,355.00
-
1 × $652.00
-
1 × $406.00
-
1 × $2,782.00
-
1 × $8,819.00
-
1 × $588.00
-
1 × $521.00
-
1 × $571.00
-
1 × $2,520.00
-
1 × $547.00
-
1 × $1,750.00
-
1 × $2,738.00
-
1 × $409.00
-
1 × $1,340.00
-
1 × $471.00
-
1 × $3,012.00
-
1 × $1,510.00
-
1 × $8,510.00
-
1 × $2,533.00
-
1 × $709.00
-
1 × $8,725.00
-
1 × $15,311.00
-
1 × $506.00
-
1 × $3,218.00
-
1 × $657.00
-
1 × $1,753.00
-
1 × $1,081.00
-
1 × $1,642.00
-
2 × $788.00
-
1 × $2,452.00
-
1 × $4,211.00
-
1 × $2,083.00
-
1 × $1,887.00
-
1 × $25,527.00
-
1 × $1,516.00
-
1 × $496.00
-
1 × $373.00
-
1 × $435.00
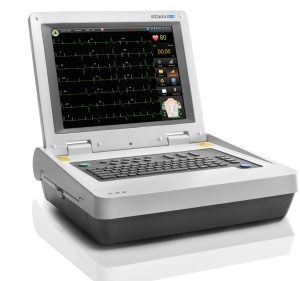
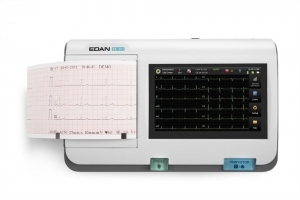
For unmatched patient convenience, the PageWriter Trim I Cardiograph enables you to quickly and easily conduct accurate, reliable 12-lead ECG tests right in your office or clinic. Trim I is a great choice for any busy clinical setting committed to lowering costs, improving efficiency, and delivering a high level of patient care.
Features and Benefits
- Philips’ most economical cardiograph with best-in-class Philips 12-Lead Algorithm
- Generates detailed, printed ECGs at any point of care — complete with optional interpretation which highlights areas for review
- Offers a compact size that’s easy to learn and use
Technical Specifications
- Patient Interface Module: Remove, microprocessor-controlled, digital module provides 5uV resolution
- Sampling Rate: 4000 samples per second per lead wire
- Severity and Interpretive statements: 8 severity levels, 558 interpretive statements
- Size: 2 lines X 40 characters
- Resolution: 5 x 7 dot/character
- Technology: LCD
- Leads off advisory: displays the label of any loose or disconnected leads/electrodes
- Heart Rate: continuous display of patient heart rate
- Dimensions: 388 x 310 x 106 mm (15.3 x 12.2 x 4.2 in)
- Weight (includes battery, patient module, lead wires, alligator clips, electrode pack and paper pack): 6.95 kg (15.3 lb)
- IEC 60601-1:1988 +A1:1991 +A2:1995 General Requirement for Safety
- IEC 60601-1-2:2001 General Requirements for Safety for Electromagnetic Compatibility
- IEC 60601-2-25:1993 + A1:1999 Safety of Electrocardiographs
- IEC 60601-2-51:2003 Particular requirements for the safety and necessary performance of electrocardiographs
- UL 2601-1:L1997 US General Requirements for Safety
- CAN/CSA-C22.2 No. 601.1-M90 S1:1994 B:1996,AAMI EC11 1991:Diagnostic Electrocardiographic Devices
- CISPR11:1997 + A1:1999 + A2:2002 Radio Frequency Disturbance, Limits and Methods of Test Group 1 Class B
- JIST 1202:1998 Japanese Industrial Standard for Electrocardiographs