-
1 × $593.00
-
1 × $7,744.00
-
1 × $3,820.00
-
1 × $385.00
-
1 × $458.00
-
1 × $816.00
-
1 × $1,842.00
-
1 × $2,452.00
-
2 × $623.00
-
1 × $466.00
-
1 × $941.00
-
1 × $3,704.00
-
1 × $2,492.00
-
1 × $437.00
-
1 × $1,585.00
-
1 × $1,111.00
-
1 × $772.00
-
1 × $5,036.00
-
1 × $1,255.00
-
1 × $1,010.00
-
1 × $573.00
-
1 × $2,314.00
-
1 × $391.00
-
1 × $560.00
-
1 × $1,366.00
-
1 × $570.00
-
1 × $385.00
-
1 × $1,092.00
-
1 × $415.00
-
1 × $1,078.00
-
1 × $494.00
-
1 × $1,247.00
-
1 × $2,533.00
-
1 × $3,552.00
-
1 × $1,374.00
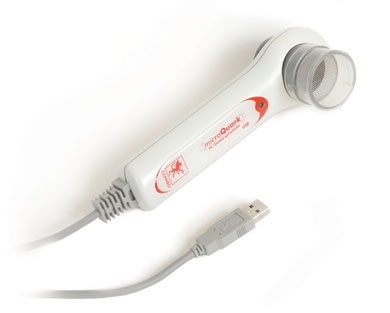
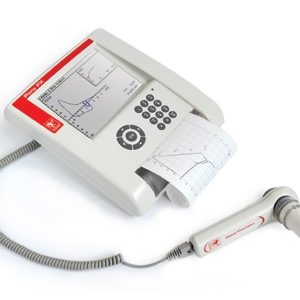
MicroQuark is the new PC spirometer designed by COSMED for lung function screening. It can be used with any PC through a USB connection. All maneuvers (FVC, SVC and MVV) are displayed in real time, so the user can monitor tests while they are performed.
Features and Benefits
- Full Spirometry testing (FVC, SVC, MVV, Pre-Post).
- Bronchial Challenge test (Metacholine and bronchial dilator).
- Automatic diagnosis according to ERS/ATS standards and COPD diagnosis (GOLD).
- Advanced software, patient database, diagnosis, real-time tests, professional prints, trends.
- Digital turbine flowmeter validated by LDS Hospital (ATS Standards).
Tests Performed
- Forced Vital Capacity (SVC-FVC)
- Maximum Voluntary Ventilation (MVV)
- Bronchial Dilator Test.
- Bronchial Challenge Test.
Measured Parameters
FVC • IVC • VC • MVV • VT • FEV1 • FEV6 • FEV1/FEV6 • FEV6/FVC • PEF • PIF • FEV1/FVC • FEF 25-75 • FEV1/VC% • %FEV1 • MEF25% • MEF50% • MEF75% • FET 100% • Lung Age • ERV • IRV • VE • Rf • ti • te • ti/t.tot • VT/ti • Best FVC • Best FEV1 • IC
Materials Included
- CD with PC software and user manual
- Turbine flowmeter
- 2 Nose clips
- Serial communication cable
- Carrying case
- Adapter for pediatric mouthpieces
- Conic mouthpiece
- 20 Adult mouthpieces
- 20 Pediatric mouthpieces
Technical Specifications
- Flowmeter: Bi-directional Digital Turbine Ш 28 mm
- Flow Range: 0.08-20l/s
- Volume range: 12 litres
- Accuracy of reading: ±2%
- Resistance: <,0.6 cmH2O/l/s @ 14l/s
- Temperature sensor: 0-50° C
- Safety: Complies with MDD (93/42 EEC),FDA 510(k) cleared (federal law restricts this device to sale by or on the order of a physician). EN 60601-1 (safety) / EN 60601-1-2 (EMC).
Warranty Information
- 3-Year Warranty
DocumentationBrochureSample Report